pH tester for food, a seemingly simple tool, holds the key to unlocking the profound mysteries of culinary creation and safeguarding the essence of life itself. Within its humble form lies the power to discern the subtle dance of acidity and alkalinity, the very balance that governs the taste, safety, and longevity of our sustenance. This exploration delves into the heart of this essential instrument, revealing its significance in the realm of food, a journey from the raw ingredients to the final, exquisite dish.
The pH tester acts as a guide, helping us navigate the complex world of food, revealing how pH levels influence taste, shelf life, and even the growth of invisible microbial worlds. Understanding pH empowers us to control processes like fermentation, where the balance of acids and bases transforms ingredients into delightful and nourishing products. Neglecting this subtle measure could lead to spoilage, diminished taste, and, most alarmingly, health risks, underscoring the critical importance of this instrument in the journey of food from farm to table.
Introduction to pH Testing in Food
Yo, foodies! Ever wonder what makes your favorite snacks safe and delish? It’s not just about the ingredients; it’s also about the invisible world of pH. Think of pH like the acidity or alkalinity meter for your grub. Understanding it is crucial for everything from keeping your food fresh to preventing a total flavor fail. Let’s dive in!A pH tester, also known as a pH meter, is a scientific instrument used to measure the acidity or alkalinity of a solution.
In the food world, it’s a game-changer. It works by measuring the concentration of hydrogen ions (H+) in a substance, giving us a number on a scale from 0 to 14. A pH of 7 is neutral (like pure water), below 7 is acidic, and above 7 is alkaline (also called basic). It’s like a food’s secret superpower, determining how it tastes, how long it lasts, and whether it’s safe to eat.
Significance of pH Levels in Food Products
pH levels have a major influence on food. They can affect the taste, shelf life, and safety of various products. The pH level determines if bacteria can grow and spoil the food.
Obtain access to chinese food clayton to private resources that are additional.
- Taste: The pH directly impacts the taste of food. Acids, with low pH levels, often give food a sour or tangy taste. Alkalines, with high pH levels, may give a bitter taste. Think about the difference between a lemon (acidic) and baking soda (alkaline).
- Example: Pickles, with their tangy flavor, achieve this through fermentation, which lowers the pH due to the production of lactic acid.
- Shelf Life: pH is a key factor in determining how long food stays fresh. Lower pH levels (more acidic) typically inhibit the growth of spoilage organisms like bacteria and mold, extending the shelf life.
- Example: Canned goods are often processed at high temperatures to kill bacteria, and their acidity (pH) is also controlled to prevent further microbial growth and spoilage during storage.
- Microbial Growth: Most bacteria and other microorganisms thrive in a narrow pH range, typically near neutrality (pH 6.5-7.5). By adjusting the pH, we can create an environment that inhibits or kills these organisms.
- Example: Fermented foods, like yogurt and sauerkraut, use beneficial bacteria to create an acidic environment that prevents the growth of harmful bacteria.
Consequences of Not Monitoring pH in Food Production
Ignoring pH in food production can lead to some seriously unpleasant consequences. It’s not just about a bad taste; it’s about public health and wasted resources.
- Health Risks: Improper pH control can allow harmful bacteria like
-Clostridium botulinum* to thrive, especially in low-acid canned foods. This can lead to botulism, a severe form of food poisoning that can be fatal. - Example: In 2007, a batch of improperly canned salsa led to a botulism outbreak, causing serious illness.
- Spoilage: Without proper pH control, food spoils faster. This can lead to unpleasant odors, changes in texture, and the growth of molds and other spoilage organisms, rendering the food inedible.
- Example: Milk that’s not properly pasteurized and stored at the right temperature will quickly turn sour due to bacterial growth, largely influenced by pH changes.
- Economic Loss: Food spoilage leads to significant economic losses. Businesses lose money on wasted ingredients, disposal costs, and potential recalls.
- Example: The US Department of Agriculture estimates that food waste costs the United States billions of dollars each year, and improper pH control is a contributing factor.
Types of pH Testers for Food
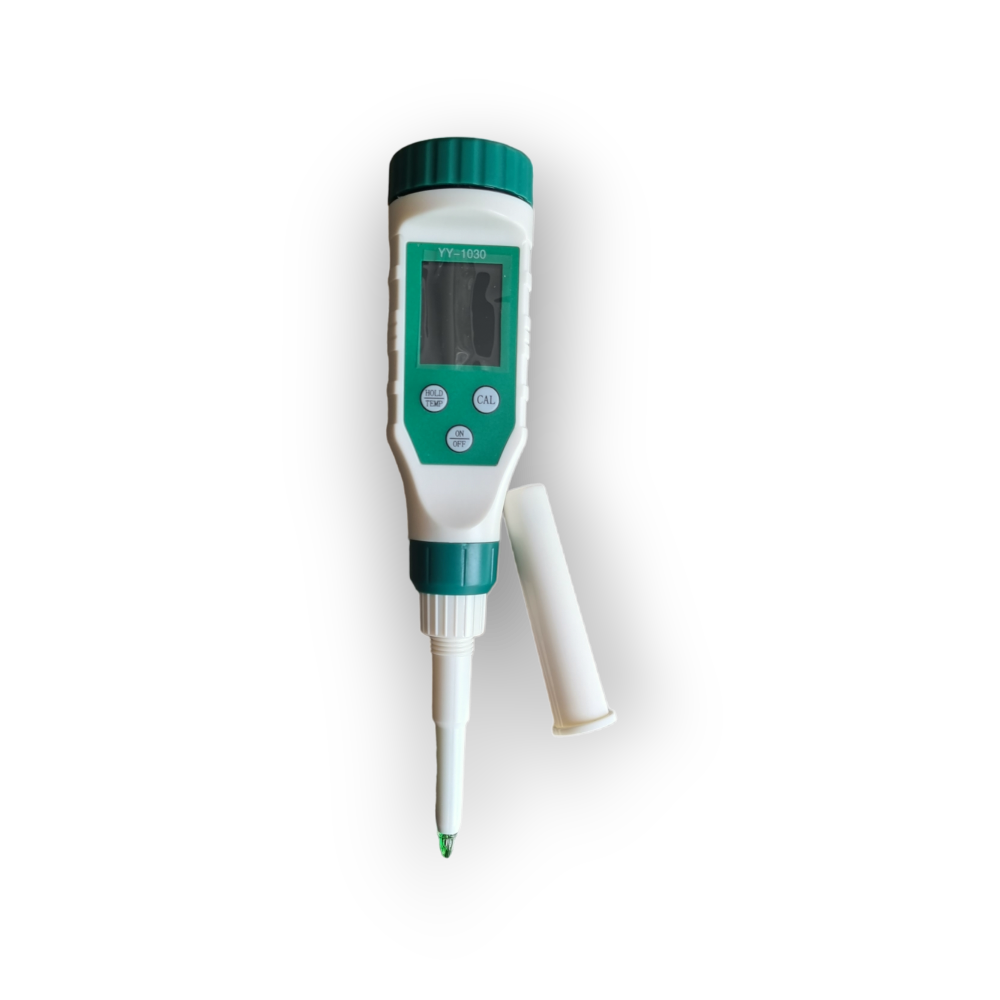
Alright foodies, now that we’ve got the lowdown on why pH matters in our culinary adventures, let’s dive into the gadgets that make it all happen! Think of pH testers as the secret agents of the kitchen, quietly keeping tabs on the acidity of your favorite dishes. From high-tech handhelds to simple test strips, there’s a pH tester for every level of food fanatic.So, let’s break down the different types of pH testers available for food applications, giving you the inside scoop on their strengths, weaknesses, and how to keep them calibrated and ready to roll.
We’ll cover the major players and the best use cases for each.
Handheld pH Meters
Handheld pH meters are like the Swiss Army knives of the pH testing world – versatile, portable, and packed with features. These are the workhorses for anyone serious about food pH, whether you’re a seasoned chef or a dedicated home cook. They offer a great balance of accuracy, convenience, and cost.
- Description: These are battery-powered devices, usually with a digital display showing the pH reading. They have a probe that’s inserted into the food sample to measure its acidity or alkalinity. Some models come with features like temperature compensation (because temperature affects pH readings), data logging (for tracking changes over time), and even Bluetooth connectivity (to sync with your phone or computer).
- Advantages: They’re super accurate, making them ideal for precise measurements. They’re portable, so you can take them anywhere. They’re relatively easy to use, and many models are designed with food safety in mind (think waterproof casings and easy-to-clean probes).
- Disadvantages: They require calibration, which adds a small step to the process. They can be a bit pricier than pH strips, but the investment is often worth it for the accuracy and features. The probes can be fragile, so handle them with care.
- Example: Imagine you’re making sourdough bread. A handheld pH meter lets you track the fermentation process, ensuring your starter is at the perfect pH for that tangy, delicious flavor. Or picture yourself testing the pH of homemade pickles to ensure they’re safe and have that perfect vinegary bite.
Benchtop pH Meters
Benchtop pH meters are the heavy hitters, the laboratory-grade equipment used for the most precise and demanding pH measurements. These are the go-to choice for food science labs, large-scale food production facilities, and anyone who needs the absolute highest level of accuracy.
- Description: These are larger, stationary units that sit on a benchtop. They usually have a larger display, more advanced features, and often come with a built-in stirrer for the sample. They’re designed for stability and accuracy, often with multiple calibration points.
- Advantages: They offer the highest level of accuracy and precision. They often have advanced features like data logging, automated calibration, and multiple probe options. They are designed for heavy use and are built to last.
- Disadvantages: They’re not portable, so they’re limited to a fixed location. They’re typically the most expensive type of pH meter. They can be more complex to operate than handheld meters, requiring some training.
- Example: A food processing plant uses a benchtop pH meter to monitor the pH of their sauces throughout the production process, ensuring consistent quality and safety. A research lab uses one to analyze the pH of different food additives to understand their impact on product shelf life.
pH Strips
pH strips are the low-tech, budget-friendly option, like the instant film cameras of the pH testing world. They’re simple to use, inexpensive, and great for quick checks.
- Description: These are paper strips impregnated with a pH-sensitive dye. When the strip is dipped into a food sample, the dye changes color, and you compare the color to a color chart to estimate the pH. They’re available in a wide range of pH scales.
- Advantages: They’re super cheap and easy to use – no batteries or calibration needed! They’re portable and disposable, making them ideal for on-the-go testing. They’re great for a quick, general idea of the pH level.
- Disadvantages: They’re less accurate than meters, providing only an approximate pH value. The color matching can be subjective, leading to some variability in readings. They’re not suitable for precise measurements or critical applications.
- Example: A home cook uses pH strips to check the acidity of their homemade jam, making sure it’s acidic enough to prevent spoilage. A student uses them for a simple experiment in a culinary class.
Comparison of pH Tester Types
Let’s get down to the nitty-gritty and compare these pH testers head-to-head. Here’s a table to break it all down:
| Feature | Handheld pH Meter | Benchtop pH Meter | pH Strips |
|---|---|---|---|
| Accuracy | High | Very High | Low |
| Ease of Use | Moderate | Moderate to High | Easy |
| Cost | Moderate | High | Low |
| Portability | High | Low | High |
| Features | Temperature compensation, data logging (often), waterproof design | Automated calibration, built-in stirrer, multiple probe options, extensive data logging | None |
| Calibration Required | Yes | Yes | No |
| Best Use Cases | Home cooking, small-scale food production, field testing | Food science labs, large-scale food production, quality control | Quick checks, home cooking (for less critical applications), educational settings |
Calibration Process for Different pH Tester Types
Calibration is like giving your pH tester a regular check-up to ensure it’s telling you the truth. It’s essential for accurate readings, and the process varies depending on the type of tester.
- Handheld and Benchtop Meters: These meters need to be calibrated using buffer solutions. Buffer solutions are liquids with a known and stable pH. You typically use at least two buffer solutions (often pH 4 and pH 7) to calibrate the meter. Some meters also require a third buffer (pH 10) for a wider range. The meter’s instructions will guide you through the process, which usually involves:
- Rinsing the probe with distilled water.
- Immersing the probe in the first buffer solution (e.g., pH 7).
- Following the meter’s instructions to calibrate at that pH point.
- Rinsing the probe again.
- Immersing the probe in the second buffer solution (e.g., pH 4).
- Calibrating at the second pH point.
- Rinsing the probe and storing it properly.
- pH Strips: pH strips don’t require calibration. You simply dip the strip into the sample and compare the color change to the color chart. However, it’s crucial to store the strips properly (in a cool, dry place) to maintain their accuracy. Always use fresh strips for the most reliable results.
- Buffer Solutions: Buffer solutions have a limited shelf life. Always check the expiration date and replace them as needed. Using expired or contaminated buffers will lead to inaccurate calibration and unreliable pH readings. Keep your buffer solutions sealed when not in use to prevent contamination and evaporation. For example, if you are testing a sample of tomato sauce, you should rinse the probe with distilled water after each test to remove any residue that could affect the next reading.
Applications of pH Testers in Food Processing
Alright, foodies and science nerds! We’ve already covered the basics of pH and the cool tools that measure it. Now, let’s dive into the real world and see how pH testers are the unsung heroes of the food industry, making sure our grub is safe, tasty, and exactly what the label says it is. From farm to table (or, more accurately, factory to fridge), pH testing is essential.
pH Testing in Food Processing Stages
pH testers aren’t just for measuring the acidity of your morning orange juice. They play a crucial role at every step of food production, keeping things in check and preventing spoilage or even worse, making you sick.Raw material inspection is the first line of defense. Before anything hits the processing line, pH testing can identify spoilage or contamination in incoming ingredients like fruits, vegetables, and meats.
This helps prevent problems from spreading throughout the whole batch. During processing, pH monitoring ensures that processes like fermentation, pickling, and acidification are working correctly. For example, the pH of yogurt needs to drop to a certain level during fermentation to develop its characteristic tang and texture. Finally, pH testing is vital for quality control of the finished product. It guarantees the food meets safety standards, maintains the desired taste, and has a long shelf life.
pH Testing in Specific Food Products
Certain foods are particularly sensitive to pH changes, and testing is absolutely critical for ensuring their quality and safety. Here’s a rundown of some key players:
- Dairy Products: Milk, yogurt, cheese, and ice cream are all vulnerable to spoilage caused by bacterial growth. pH testing ensures proper fermentation, pasteurization, and storage conditions. For instance, the pH of milk can indicate freshness. A higher pH than normal can indicate spoilage.
- Meat Products: pH levels impact the color, texture, and safety of meat. Testing during processing (like curing and smoking) helps control bacterial growth and maintain the desired quality.
- Canned Goods: Canning involves heating food to destroy harmful microorganisms. The acidity of the food is crucial to the effectiveness of this process. Low-acid foods (pH above 4.6) require higher temperatures and longer processing times to ensure safety.
- Pickled Foods: The characteristic sour taste of pickles and other pickled items comes from lactic acid produced during fermentation. pH testing verifies that enough acid has been produced to inhibit the growth of harmful bacteria and preserve the food.
- Beverages: From fruit juices to sodas, pH levels directly affect taste, stability, and safety.
- Sauces and Dressings: These often rely on specific pH levels to prevent spoilage and achieve the desired flavor profile.
Optimizing Food Processing Parameters with pH Measurements
pH isn’t just about safety; it’s also a key to achieving the perfect flavor and texture. By carefully monitoring and controlling pH, food processors can fine-tune their processes for optimal results.For example, in fermentation processes like brewing beer or making sourdough bread, pH measurements guide the timing and duration of the process.
The optimal pH range for fermentation can be critical to the production of specific flavors and the overall quality of the final product.
In cooking, pH can influence the texture and tenderness of meat. Acidic marinades, for example, can tenderize meat by breaking down proteins. Also, during cooking, pH changes can impact the color of foods.In the production of cheese, the pH level of the milk directly impacts the final product. The milk is acidified to a certain pH level, which causes the proteins to coagulate and form curds.
This process is critical to producing the desired texture and flavor.
Choosing the Right pH Tester
Alright, foodies and lab coat aficionados, choosing the right pH tester is like picking the perfect avocado – it’s gotta be just right for the job! Get the wrong one, and your guacamole could end up tasting like sadness. This guide will help you navigate the wild world of pH testers and find the one that’s your perfect match, ensuring your food is safe, delicious, and up to snuff.
Decision-Making Guide for Selecting a pH Tester
Selecting a pH tester isn’t a one-size-fits-all situation. Several factors influence the ideal choice. Consider these points when making your decision to ensure the tester aligns with your specific needs.
- Type of Food Being Tested: This is the cornerstone of your decision. Different food matrices (the “stuff” your food is made of) present unique challenges.
- Liquids (e.g., juices, sauces, milk): A standard pH tester with a glass electrode will generally suffice. These are easy to use and provide reliable readings. Think of it as the classic burger – reliable and gets the job done.
- Semi-Solids (e.g., yogurt, jams, dough): You’ll need a tester with a more robust probe, perhaps a spear-tip or a flat-surface electrode. These probes can penetrate thicker substances without breaking. Imagine a steak – needs a good knife to cut through.
- Solids (e.g., cheese, meat): For solid foods, a penetration probe is crucial. This type of probe allows for direct insertion into the food. Think of it as a good drill – getting straight to the point.
- Desired Level of Accuracy: Are you aiming for precision or a general idea? This will determine the type of tester and the features you need.
- Laboratory-Grade Testers: These offer the highest accuracy, often to the hundredth or thousandth of a pH unit. They’re like a luxury car – high performance, but also a bigger investment. They often have multiple calibration points and advanced temperature compensation.
- Field Testers: These are portable and offer good accuracy, usually to the tenth of a pH unit. Think of them as a reliable pickup truck – good for various tasks and easy to transport.
- Pocket Testers: These are the simplest and least expensive, providing a quick reading. They’re like a bicycle – good for basic needs, but may not be suitable for demanding tasks.
- Frequency of Testing: How often will you be testing? This impacts the type of tester you choose.
- Frequent Testing (e.g., a food production line): A durable, easy-to-calibrate tester is essential. Consider a benchtop model or a ruggedized handheld model. This is like having a reliable employee – always there when you need them.
- Infrequent Testing (e.g., home use, occasional quality checks): A simple, portable tester may be sufficient. This is like a seasonal helper – good for the occasional project.
- Probe Type and Considerations: The probe is the heart of the pH tester, and the right type is critical.
- Glass Electrode: The most common type, suitable for liquids.
- Spear-Tip Electrode: Designed for semi-solids.
- Flat-Surface Electrode: Ideal for measuring the pH of surfaces or small samples.
- Penetration Probe: Specifically designed for solid foods.
- Consider probe material: Some probes are more resistant to certain chemicals or temperatures than others. Think about the food’s composition and how it might interact with the probe.
- Temperature Compensation: Food temperature significantly affects pH readings.
- Automatic Temperature Compensation (ATC): This feature automatically adjusts the pH reading based on the food’s temperature, ensuring accuracy. It’s like having GPS in your car – always making corrections for you.
- Manual Temperature Compensation: You’ll need to manually enter the food’s temperature. This is like using a map and compass – requires more user input.
- Calibration: Regular calibration is vital for accurate readings.
- Calibration Solutions: You’ll need calibration solutions (buffers) to calibrate your pH tester. These are like the “training wheels” for your tester.
- Number of Calibration Points: More calibration points generally lead to greater accuracy. Lab-grade testers often use multiple points (e.g., pH 4, 7, and 10).
- Calibration Frequency: Follow the manufacturer’s recommendations for calibration frequency. Regular calibration ensures the tester remains accurate over time.
- Additional Features: Consider these extras to make your life easier.
- Data Logging: Allows you to record pH readings over time.
- Backlit Display: Useful in low-light conditions.
- Waterproof/Water-Resistant Design: Essential for use in wet environments.
- Ease of Use: Look for a tester with clear instructions and a user-friendly interface.
Remember, the best pH tester is the one that best meets your specific needs. Consider all these factors carefully to make the right choice and keep your food safe and delicious.
Methods and Procedures for pH Testing
Alright foodies, let’s get down to the nitty-gritty! Knowing the pH of your eats isn’t just for lab coats and science fairs anymore. It’s crucial for everything from food safety to understanding how that sourdough gets its signature tang. So, grab your pH testers (the ones you chose in the previous sections, of course!), and let’s learn how to make some accurate measurements.
Step-by-Step Procedure for pH Testing
Getting a reliable pH reading is like baking a perfect cake: follow the recipe, and you’ll get delicious results. Here’s a straightforward procedure:
- Calibration is Key: Before you even think about your food sample, calibrate your pH meter. This is super important! Most meters come with calibration solutions (pH 4, pH 7, and sometimes pH 10). Dip the electrode into each solution, one at a time, and follow your meter’s instructions to adjust the readings. Think of it like tuning a guitar before you start playing a gig.
- Sample Prep: Get your sample ready. We’ll dive deeper into sample prep for different food types later, but the basic idea is to get a representative and homogenous sample. Make sure your sample is at a stable temperature, ideally room temperature, because temperature can affect pH readings.
- Rinse and Rinse Again: Before you measure your food, rinse the electrode with distilled or deionized water. This removes any residue from the calibration solutions or the previous sample. Then, gently blot the electrode dry with a soft, lint-free cloth.
- Dip and Swirl: Carefully immerse the electrode into your food sample. Make sure the sensing bulb is fully submerged. Gently swirl the electrode in the sample. This helps the electrode equilibrate and gives you a more accurate reading. Avoid hitting the bottom of the container, as this could damage the electrode.
- Wait for the Reading: Allow the pH meter to stabilize. This might take a few seconds to a minute, depending on the meter and the sample. The display will usually show a stable reading once it’s ready. Some meters have a “hold” function that freezes the reading.
- Record the Result: Note down the pH value. Be sure to include the temperature at which the measurement was taken. This is important for traceability and for comparison with other readings.
- Rinse and Store: After each measurement, rinse the electrode thoroughly with distilled or deionized water and blot it dry. Store the electrode according to the manufacturer’s instructions, usually in a storage solution to keep it hydrated and functioning properly.
Sample Preparation Techniques
Different foods require different prep techniques. Imagine trying to measure the pH of a whole apple versus apple juice – totally different approaches! Here’s how to prep some common food types:
- Liquids (Juices, Milk, Yogurt): These are usually pretty straightforward. Just ensure the sample is well-mixed before taking a reading.
- Semi-Solids (Purees, Sauces, Jams): These might need some mixing or homogenization. Use a blender or food processor to create a smooth, uniform sample.
- Solids (Meats, Cheeses, Fruits, Vegetables): This is where it gets interesting. You’ll need to extract the liquid from the food. One common method is to blend a portion of the solid food with distilled or deionized water. The ratio of food to water is crucial. A common ratio is 1:10 (one part food to ten parts water).
- Dilution is Sometimes Necessary: For highly acidic or alkaline foods, dilution can bring the pH into the meter’s measurable range. However, be careful with dilution, as it can alter the true pH of the food. Always document the dilution factor used.
Example: For meat, grind or chop a 10-gram sample, add it to 100 ml of distilled water, and blend until you get a homogeneous slurry. Let it sit for a few minutes to allow the solids to settle before measuring the pH of the liquid portion.
Example: If a food sample is extremely acidic, dilute it with a known amount of distilled water. If you dilute a sample 1:1 (one part sample, one part water), multiply the pH reading by a factor to account for the dilution.
Maintaining and Cleaning pH Testers
Taking care of your pH tester is like taking care of your favorite kitchen knife: it keeps it sharp and reliable. Regular maintenance ensures accurate and long-lasting performance.
- Cleaning is a Must: After each use, rinse the electrode thoroughly with distilled or deionized water to remove any food residue. Use a mild cleaning solution (like a specialized electrode cleaning solution, or a diluted detergent solution) periodically to remove stubborn deposits. Avoid harsh chemicals that can damage the electrode.
- Storage Matters: Always store the electrode in its appropriate storage solution. This solution keeps the glass bulb hydrated and ready for use. Never let the electrode dry out.
- Calibration is Regular: Calibrate your pH meter regularly, even if you haven’t used it. The frequency depends on how often you use it, but monthly calibration is a good rule of thumb.
- Inspect the Electrode: Check the electrode for any cracks, scratches, or damage. If you see any, replace the electrode immediately.
- Follow the Manual: Always refer to the manufacturer’s instructions for specific cleaning and maintenance procedures. They know their equipment best!
Interpreting pH Readings in Food
Alright, foodies, let’s get real about pH. It’s not just some fancy science term; it’s the secret sauce behind safe and delish eats. Understanding those pH readings is like having a superpower in the kitchen or the food processing plant, letting you know if your grub is good to go or a potential food safety hazard. So, buckle up; we’re about to decode the numbers game.Interpreting pH readings is crucial for maintaining food safety and ensuring product quality.
It’s the difference between a perfectly preserved pickle and a potential botulism nightmare. The pH value tells us how acidic or alkaline a food product is, which directly impacts microbial growth, texture, flavor, and shelf life.
pH and Food Safety Standards
The pH scale, ranging from 0 to 14, helps us understand the acidity or alkalinity of a food product. A pH of 7 is neutral; anything below 7 is acidic, and anything above 7 is alkaline (or basic). Food safety guidelines often use a pH of 4.6 as a critical cutoff. Foods with a pH above 4.6 are considered “low-acid” and require more stringent processing to prevent the growth of harmful bacteria like
Clostridium botulinum*, which can cause botulism, a potentially fatal illness.
Here’s the deal, food safety pros have laid down some ground rules. The FDA (Food and Drug Administration) and other regulatory bodies have established acceptable pH ranges for different food products to minimize foodborne illness risks and maintain quality standards. Here’s a breakdown of the pH ranges, alongside the relevant regulatory guidelines:
| Food Product | Acceptable pH Range | Regulatory Guidelines | Notes |
|---|---|---|---|
| Canned Tomatoes | < 4.6 | FDA – 21 CFR Part 113 (Thermally Processed Low-Acid Foods Packaged in Hermetically Sealed Containers) | Tomatoes are naturally acidic. Proper canning ensures acidity inhibits bacterial growth. |
| Pickles | < 4.6 | FDA – 21 CFR Part 114 (Acidified Foods) | Fermentation or acidification lowers the pH, preserving the cucumbers and preventing spoilage. |
| Fruit Jams and Jellies | < 3.5 | FDA – Generally Recognized as Safe (GRAS) | High sugar content combined with low pH inhibits microbial growth, contributing to shelf stability. |
| Sausage (Fermented) | < 5.3 | USDA – FSIS (Food Safety and Inspection Service) | Fermentation by lactic acid bacteria lowers pH, contributing to flavor and preservation. |
The above table is a general guide. Always consult specific regulations for the products you are working with, as these standards can be product-specific and subject to change.
Actions for Out-of-Range pH Readings
Okay, so what happens when the pH reading throws a curveball? If the pH of a food product falls outside the acceptable range, you’ve got a problem, and it’s time to spring into action. Ignoring these deviations is like playing Russian roulette with your customers’ health.
- Immediate Action: The first step is to isolate the affected product. Prevent it from entering the distribution chain to avoid potential health risks.
- Investigation: Determine the root cause of the out-of-range pH. Possible causes include incorrect ingredient ratios, equipment malfunctions, or process errors. Conduct a thorough investigation to identify the source of the problem.
- Corrective Measures: Implement corrective actions based on the investigation’s findings. This may involve adjusting ingredients, recalibrating equipment, or modifying processing procedures.
- Re-testing: After implementing corrective measures, re-test the product’s pH to ensure it falls within the acceptable range.
- Documentation: Maintain detailed records of all pH readings, investigations, corrective actions, and re-testing results. This documentation is critical for regulatory compliance and traceability.
- Reporting: Depending on the severity of the deviation and the product type, you may need to report the issue to the relevant regulatory agencies, such as the FDA or USDA. Failure to report can result in penalties and damage to your brand’s reputation.
Remember: A pH reading outside the acceptable range is a red flag. Take it seriously, investigate the cause, and implement corrective actions to ensure food safety and quality.
Troubleshooting Common Issues: Ph Tester For Food
Alright, foodies and science nerds! Even the coolest pH testers can throw a curveball sometimes. Think of it like your favorite food processor – sometimes it just won’t chop that onion right, right? In the world of pH testing, things can get a little wonky, leading to inaccurate readings and potential food safety headaches. But don’t sweat it! We’re about to dive into the most common issues and how to fix ’em, so you can keep your food game strong.
Inaccurate Readings and Probe Malfunctions
Sometimes, your pH tester might start giving you readings that are totally off, like telling you your lemonade is super alkaline when it’s clearly puckering your lips. Or maybe the probe itself just stops working altogether. These issues can stem from several factors, including probe contamination, improper calibration, or even the probe’s age.
- Probe Contamination: Food particles, oils, and other substances can gunk up the probe’s sensitive glass bulb, leading to inaccurate readings. It’s like trying to read a book through a smeared window.
- Improper Calibration: Calibration is like tuning your instrument. If your tester isn’t calibrated correctly, it’s going to be way off the mark. Think of it like setting your oven temperature wrong.
- Probe Age and Damage: Over time, the probe’s glass bulb can become etched or cracked, or the internal components might degrade. This can mess with its ability to measure pH accurately.
- Buffer Solution Problems: Using expired or contaminated buffer solutions will throw off the calibration, leading to incorrect readings.
- Temperature Fluctuations: Significant temperature changes can affect the accuracy of pH measurements. Always ensure the sample and the tester are at a similar temperature.
Troubleshooting Tips for Resolving Issues
Okay, so your pH tester is acting up. Don’t panic! Here’s how to get things back on track, step-by-step, like a well-choreographed dance.
- Cleaning the Probe: This is your first line of defense. Rinse the probe with distilled water after each use and gently wipe it with a soft cloth. For stubborn contaminants, use a mild cleaning solution specifically designed for pH probes. Avoid harsh chemicals, which can damage the probe.
- Calibrating the Meter: Calibration is key! Use fresh, high-quality buffer solutions with known pH values (typically pH 4, 7, and 10). Follow the manufacturer’s instructions carefully. Most meters have a simple calibration process.
- Checking the Buffer Solutions: Make sure your buffer solutions haven’t expired and haven’t been contaminated. Always store them properly and replace them regularly. Check the expiration date on the bottle, and if it’s past, toss it!
- Inspect the Probe: Examine the glass bulb for any cracks, chips, or cloudiness. If you see damage, it’s time for a new probe.
- Check the Batteries: A low battery can cause inaccurate readings. Replace the batteries if necessary.
- Temperature Compensation: Ensure the meter has been set up correctly for temperature compensation, especially when testing samples at different temperatures.
Troubleshooting Flowchart for pH Tester Issues
Let’s visualize this process. Imagine a flowchart like a treasure map, guiding you to the solution.
Flowchart Description:
The flowchart begins with the “Inaccurate pH Reading?” question. If the answer is “Yes,” the flowchart proceeds to “Clean Probe?” If the answer is “Yes,” the flowchart leads to “Calibrate Meter?” If the answer is “Yes,” the flowchart directs to “Test with Known Solution?”. If the reading is still incorrect, the flowchart directs to “Replace Probe?”. If the reading is correct, the flowchart goes to “Return to Testing”.
If the answer to “Clean Probe?” is “No,” the flowchart directs to “Calibrate Meter?”. If the answer to “Inaccurate pH Reading?” is “No,” the flowchart goes to “Is the Probe Damaged?”. If the answer is “Yes,” the flowchart leads to “Replace Probe?”. If the answer is “No,” the flowchart goes to “Return to Testing”.
Flowchart Key Steps:
1. Start
Inaccurate pH Reading?
2. Clean Probe? (Rinse with distilled water and clean gently).
3.Calibrate Meter? (Using fresh buffer solutions).
4. Test with Known Solution? (Verify with a known standard).5. Is the Probe Damaged? (Inspect for cracks, chips, or cloudiness).
6. Replace Probe?(If damaged).
7. Return to Testing (If issues are resolved).
Regulations and Standards
Alright, foodies! So, you’ve mastered the art of pH testing, from the fancy digital meters to the classic litmus strips. But knowing the numbers is only half the battle. The real game changer? Understanding how those pH readings play a crucial role in keeping our food safe and, you know, not making us sick. Think of regulations and standards as the ultimate rulebook for food safety, and pH testing is like the secret weapon to staying in compliance.
Let’s dive in and break down the essential ingredients for a successful (and safe) food operation.Basically, the FDA, USDA, and other regulatory bodies are the ultimate food police, and pH is one of their favorite tools. They’re not just being control freaks; they’re protecting us from the nasty stuff that can ruin a good meal and, worse, our health. These regulations and standards dictate how we handle, process, and store food to minimize the risk of foodborne illnesses.
pH testing is a critical piece of this puzzle.
Relevant Food Safety Regulations and Standards
The world of food safety is full of acronyms and standards, but here are a few of the big players you need to know:
- FDA (Food and Drug Administration): The FDA sets the standards for most food products sold in the US. They have specific regulations related to pH for low-acid canned foods, acidified foods, and other processed foods. Their regulations are detailed in the Code of Federal Regulations (CFR).
- USDA (United States Department of Agriculture): The USDA regulates meat, poultry, and egg products. They have their own set of standards, often overlapping with the FDA’s, focusing on ensuring the safety of these products.
- HACCP (Hazard Analysis and Critical Control Points): HACCP is a food safety management system that identifies and controls hazards throughout the food production process. pH testing is often a critical control point in a HACCP plan.
- FSMA (Food Safety Modernization Act): This act, passed in 2011, gives the FDA more authority to regulate the food industry and focuses on preventing food safety problems rather than reacting to them. FSMA emphasizes preventive controls, and pH testing plays a significant role in these controls.
- Codex Alimentarius: This is a collection of internationally recognized standards, codes of practice, guidelines, and other recommendations relating to foods, food production, and food safety. It’s a global standard that many countries use as a basis for their own regulations.
Role of pH Testing in Ensuring Compliance
So, how does pH testing actually help you play by the rules? It’s all about controlling the growth of dangerous microorganisms.
- Controlling Bacterial Growth: The pH level of food significantly affects the growth of bacteria, yeast, and molds. Many harmful bacteria, like
-Clostridium botulinum* (the one that causes botulism in canned goods), thrive in low-acid environments (pH above 4.6). By monitoring pH, you can ensure that your food is acidic enough to prevent these bacteria from multiplying. - Acidified Foods: Foods that are naturally low in acid (like vegetables) can be made safe through acidification. The FDA defines acidified foods as those with a finished equilibrium pH of 4.6 or below. pH testing is essential to ensure that the acidification process is effective.
- Low-Acid Canned Foods: Canned foods that are not acidified must be processed using heat to kill bacteria. However, the heat treatment is less effective if the pH is not controlled. pH testing is crucial to determine the correct heat treatment time and temperature.
- HACCP Implementation: As mentioned earlier, pH testing is often a critical control point in a HACCP plan. If pH is not within the acceptable range, it can indicate a potential hazard. This can trigger corrective actions, like adjusting processing parameters or discarding the product.
- Shelf-Life Determination: pH can also impact the shelf life of food products. By understanding the pH of a product, you can predict how long it will remain safe and of acceptable quality.
For example, let’s say you’re making pickles. The FDA requires a pH of 4.6 or lower to prevent the growth of harmful bacteria. If your pH readings consistently show a higher pH, you know your pickling process isn’t working and needs adjustment (more vinegar, anyone?).
Importance of Accurate Record Keeping, Ph tester for food
Think of record-keeping as the paper trail that proves you’re following the rules. Accurate records of pH measurements are not just a good practice; they’re a legal requirement. These records are crucial for traceability and auditing.
- Traceability: In case of a foodborne illness outbreak, authorities need to trace the source of the problem. Detailed pH records can help pinpoint where things went wrong in the production process.
- Auditing: Food facilities are regularly audited by regulatory agencies to ensure compliance. Auditors will review your pH records to verify that you’re following the established procedures and maintaining the required pH levels.
- Documentation of Corrective Actions: If a pH reading is out of the acceptable range, you need to take corrective action (e.g., adjusting the recipe or processing time). You must document these actions in your records, including the date, time, pH reading, and the corrective action taken.
- Preventive Controls: Consistent and accurate pH record-keeping allows you to identify trends and potential problems before they lead to a crisis. For instance, you might notice a gradual increase in pH readings over time, which could indicate a problem with your equipment or ingredients.
- Protecting Your Business: Accurate records can protect your business from legal liabilities in case of a food safety issue. They demonstrate that you took the necessary steps to ensure food safety.
| Date | Time | Product | pH Reading | Acceptable Range | Action Taken | Initials |
|---|---|---|---|---|---|---|
| 2024-07-08 | 9:00 AM | Pickled Cucumbers | 3.8 | < 4.6 | OK | J.S. |
| 2024-07-08 | 2:00 PM | Pickled Cucumbers | 4.7 | < 4.6 | Added more vinegar | J.S. |
| 2024-07-08 | 2:30 PM | Pickled Cucumbers | 4.5 | < 4.6 | OK | J.S. |
This simple log shows the date, time, product, pH reading, acceptable range, and any action taken. You can see the importance of recording the actions taken when the reading was out of range.In a nutshell, understanding and adhering to food safety regulations and standards, along with meticulous pH testing and record-keeping, is non-negotiable for any food business. It’s not just about staying out of trouble; it’s about protecting your customers, your reputation, and your business.
Future Trends in pH Testing
Alright, foodies and tech enthusiasts, buckle up! The world of pH testing is about to get a serious glow-up. We’re moving beyond the classic litmus test and dipping into a future where data streams and smart sensors rule the kitchen (and the factory). Think of it like this: we’re trading in our flip phones for the latest iPhone – the evolution is real, and it’s gonna change how we ensure our food is safe and delicious.
Emerging Technologies in pH Testing
The future of pH testing is all about ditching the manual labor and embracing automation. Imagine a world where pH readings are taken in real-time, without human intervention, providing a constant stream of data that can be analyzed and acted upon instantly.
- Wireless Sensors: These tiny marvels are the game-changers. Picture small, robust sensors that can be placed directly in food processing lines, inside storage containers, or even embedded in food products themselves. They constantly monitor pH levels and transmit the data wirelessly to a central hub. This eliminates the need for manual sampling and testing, providing continuous monitoring and allowing for immediate adjustments to the process if necessary.
For example, imagine a wireless sensor in a vat of yogurt during fermentation; it would continuously send pH readings to a control system, allowing for precise adjustments to temperature and culture addition to achieve the perfect tang.
- Automated Systems: These systems take the concept of automation to the next level. They incorporate robotic arms, automated sample collection, and sophisticated analytical software. The robotic arms collect samples, the system calibrates the pH meter, takes the measurement, and logs the data, all without any human involvement. This significantly reduces the risk of human error and speeds up the testing process.
Imagine an automated system in a large food manufacturing plant, where thousands of samples need to be tested daily. This system would drastically increase efficiency and ensure consistency.
Potential Benefits of Advanced pH Testing
The shift towards these new technologies offers a plethora of advantages, promising a new era of food safety and quality control. The benefits extend beyond just convenience; they’re about optimizing every aspect of food production.
- Increased Efficiency: Wireless sensors and automated systems significantly reduce the time and labor required for pH testing. Continuous monitoring eliminates the need for periodic sampling, and automated systems streamline the entire process. This leads to faster production cycles and reduced operational costs. For instance, a food processing plant using automated pH testing could potentially increase its output by 15-20% due to faster testing and real-time process adjustments.
- Enhanced Accuracy: Automated systems minimize the risk of human error, such as incorrect calibration or improper sample handling. Wireless sensors provide consistent and reliable data, eliminating the inconsistencies associated with manual readings. This ensures more accurate and reliable pH measurements, leading to better quality control.
- Improved Data Collection and Analysis: These technologies generate a wealth of data that can be used for process optimization and predictive maintenance. Data collected from wireless sensors and automated systems can be analyzed to identify trends, predict potential problems, and optimize production parameters. For example, by analyzing pH data over time, food manufacturers can identify subtle shifts in fermentation processes and make adjustments to ensure consistent product quality.
The Next 5-10 Years: A Tech-Forward Future
So, what does the future hold? Let’s get real: the next decade will witness a dramatic transformation in pH testing. We’re talking about a complete integration of technology into every stage of food production.
- Current State: Currently, we see a mix of traditional methods and emerging technologies. Manual pH meters are still widely used, but wireless sensors and automated systems are gaining traction, especially in larger food processing plants. Data collection is often manual or semi-automated, with some companies using spreadsheets or basic data logging software.
- Future Outlook (5-10 Years): In the next 5-10 years, we can expect to see:
- Ubiquitous Wireless Sensors: Wireless sensors will become the standard, integrated into nearly every aspect of food production, from farm to table. These sensors will be smaller, more durable, and more affordable.
- AI-Powered Analysis: Artificial intelligence (AI) and machine learning will be used to analyze the vast amounts of data generated by these sensors. AI algorithms will identify patterns, predict potential problems, and recommend real-time adjustments to the food production process.
- Smart Packaging: pH sensors will be embedded in food packaging, providing consumers with real-time information about the freshness and safety of their food. Imagine a yogurt container with a built-in pH sensor that changes color to indicate spoilage.
- Cloud-Based Data Management: All data will be stored in the cloud, allowing for easy access, analysis, and sharing across the entire supply chain. This will improve transparency and traceability, ensuring food safety from start to finish.
Concluding Remarks
As we conclude our exploration, we realize the pH tester for food is more than a mere device; it is a portal to understanding the very essence of food. It guides us toward the delicate balance between safety and flavor, guiding us to a world of culinary creation and food safety. By mastering its use, we embrace the responsibility of ensuring the health and well-being of ourselves and those we serve, embracing a deeper connection to the food that nourishes our bodies and souls.
Embrace the wisdom of the pH tester, and may your culinary journey be one of enlightenment and delight.


